Download Broschure «Key Milestones»
Inhalte
30 years of Frontiers in Drug Discovery and Development
The history of drug discovery and development goes back several thousand years.It started with the use of herbals in China and India. Later there was evidence of medicinal practice in Egypt. Hippocrates in Greece started to transform medicine from art to science. The foundation of scientific medicine was then developed over two thousand years and many discoveries and achievements came out of Basel. Modern drug discovery started to emerge by the end of the 20th century with the arrival of organic chemistry, the discipline of pharmacology and germ theory. Later industrial technology made it possible to manufacture high quality medicines. By introducing genomics the Human Genome Project enabled advances in molecular and genomic medicine in the year 2000.
Tremendous progress occurred in the last 30 years, but we still have a limited understanding of disease pathology and progression. Cutting-edge technologies emerged, e.g. as humanized models in toxicology or gene sequencing. These help to predict and personalize the clinical success of drug candidates. Algorithms, machine learning, artificial intelligence and other in silico tools assist to study molecules in a dynamic state, even within a single cell. High-throughput technologies and digital devices produce an exponential amount of data. Hence it is important that such data is transformed to high quality information and subsequently turned into actionable knowledge. In all parts of this process, skills and talents of the individuals along the value chain are key. Our aim is to support research and educate professionals in drug discovery and development, especially at the interface of disciplines.

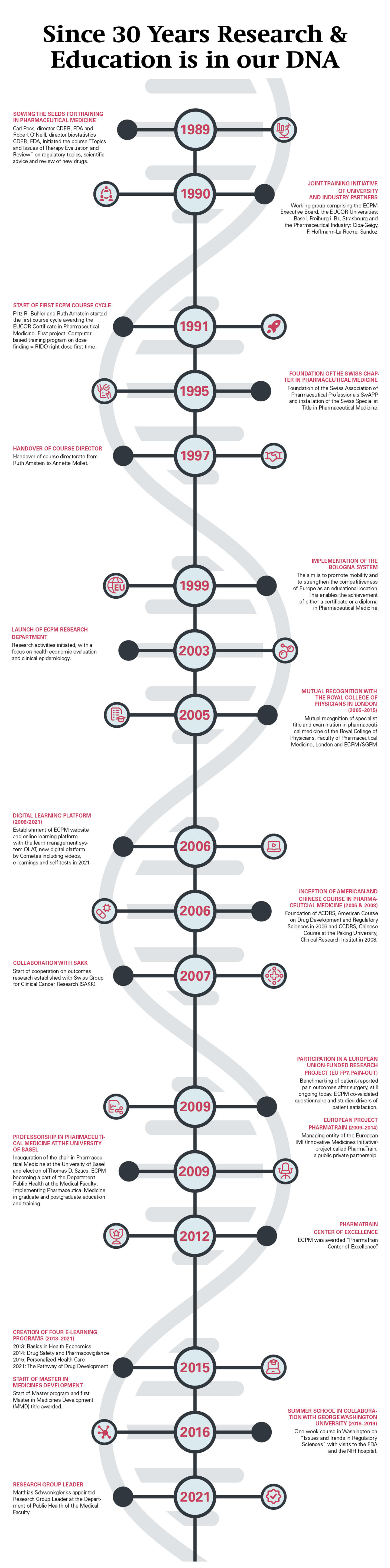
Highlights and Milestones in the history of ECPM
Robert O’Neill, head of biostatistics at FDA came 1989 to Basel with the idea to develop a course in regulatory topics. The focus was to inform European scientists about how the U.S. Food and Drug Administration (FDA) provides advice to sponsors of clinical studies and how FDA evaluates the evidence of the safety and efficacy of new drugs. Since Basel is the European center of pharmaceutical research and development, Fritz R. Bühler had the idea to enhance the university-industry relationships by offering high quality education and training on a neutral, academic ground.
After the big success of the initial course, Fritz R. Bühler and Ruth Amstein started the ECPM course in 1991 and developed a two-years curriculum – the ECPM course was born. The course was part of the postgraduate training at the University of Basel within the medical faculty and partner of EUCOR, the European campus consisting of the Universities Basel, Freiburg i.Br and Strasbourg. Until 1999 the successful course led to an EUCOR certificate which after the introduction of the Bologna system in 2000 was upgraded to a postgraduate diploma.
In 1997 Annette Mollet joined ECPM as the new course director. From 2009 to 2014 Fritz R. Bühler, Thomas D. Szucs, Susanne Daniel and Annette Mollet led the IMI, Innovative medicines Initiative, project – a public/private partnership called PharmaTrain. Through this European academic network the ECPM training platform was taken to the next level to offer a postgraduate master title. In 2006 and 2008 the University of Beijing and the subsidiary of the University of San Francisco in Washington adopted the course concept.
In 2003 the research group was established under the leadership of Matthias Schwenkglenks, with a research focus on health economic characteristics, cost-benefit implications and the efficient use of pharmaceuticals and other healthcare interventions in Switzerland and internationally. Long-standing cooperation on outcomes research with the Swiss Group for Clinical Cancer Research (SAKK) and collaborations within the University Basel and Zurich and the pharmaceutical industry were established. One of the most important milestones was the inauguration of the professorship in pharmaceutical medicine and the election of Thomas D. Szucs at the University of Basel in 2009 that paved the way for ECPM to become a full university institute at the department public health within the medical faculty. The research department evolved over time and consists today of seven research scientists and a number of PhD and master students
Since then we are striving to implement teaching in drug development, health economics and policy for the undergraduate students and to continue to offer cutting edge courses by implementing digital course tools and offers. Thanks to our local and international collaboration with partners from the pharmaceutical industry, academia and governmental/regulatory authorities, our research and training remain an important support for modern drug development and informed and transparent health policy decision making.

ECPM Education & Training
Pharmaceutical Medicine
Pharmaceutical Medicine is the medical scientific discipline concerned with the discovery, development, evaluation, registration, monitoring and medical aspects of marketing of medicines for the benefit of patients and the public’s health.
IFAPP, International Federation of Associations of Pharmaceutical Physicians and Pharmaceutical Medicine, IFAPP, founded in 1975.
Mission
Our mission is to establish the best international training platform that provides and enhances the knowledge, expertise and skills required to perform modern discovery, development, regulation and marketing of medical products.
ECPM Course in a Nutshell


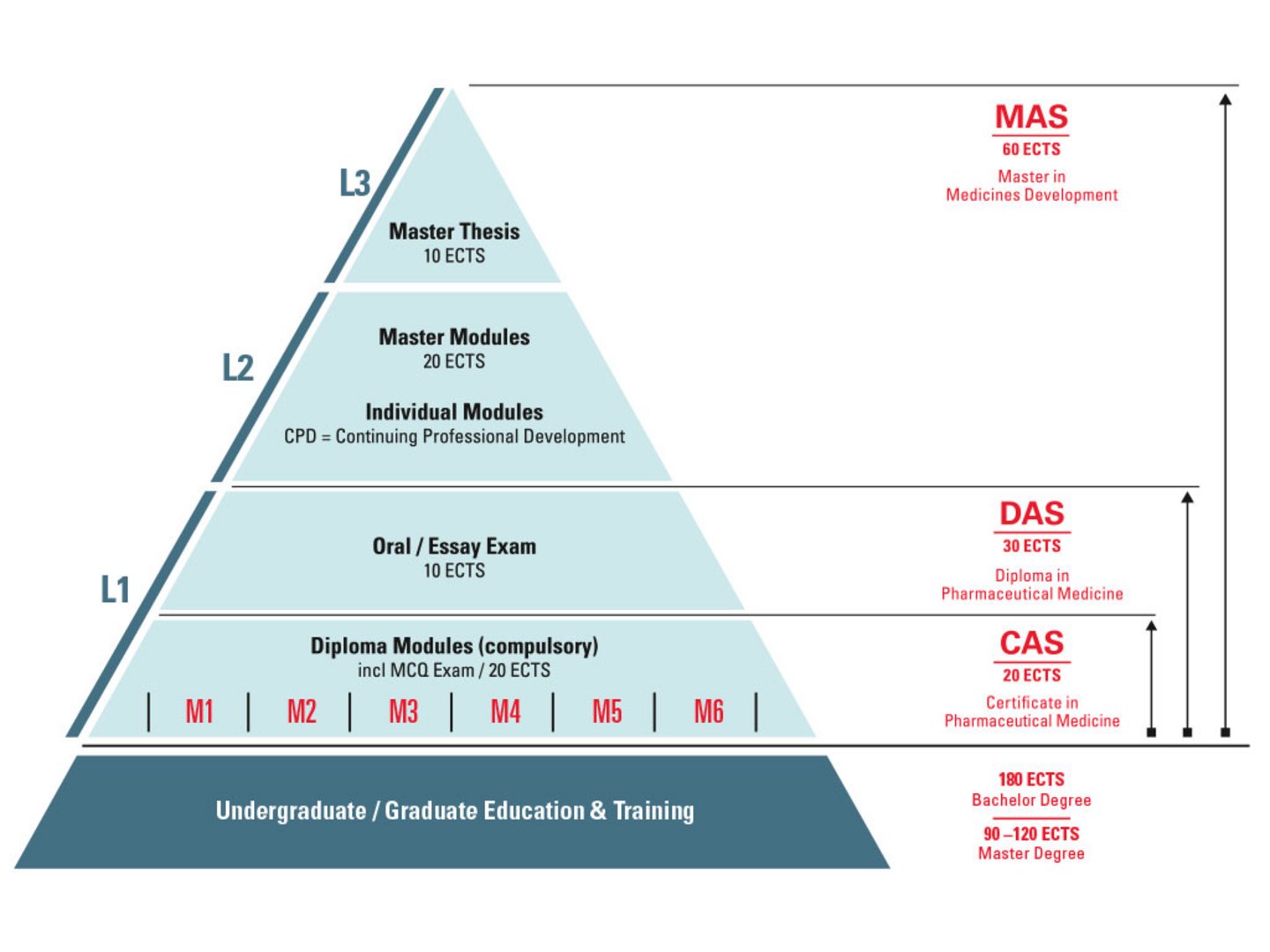
Postgraduate title system: The first level [L1] represents the Certificate/Diploma of Advanced Studies in Pharmaceutical Medicine including six mandatory basic modules (CAS 20 ECTS / DAS 30 ECTS). The diploma can be supplemented on a second level [L2] with CPD short courses and a thesis to achieve a Master of Advanced Studies [L3] (60 ECTS). The diploma covers also the theoretical part to apply for the Swiss specialist title in pharmaceutical medicine.
ECPM Research
Key Areas of Expertise:
- Pharmacoeconomics and health economics
- Model-based and trial-based cost-effectiveness analysis
- Behavioral economics
- Epidemiology
- Outcomes research
- Clinical and observational study designs
- Biostatistic
- Analysis of real-world data
Example Areas of Activity:
- Variation in healthcare utilization
- Medication utilization in Switzerland
- Approaches to health technology assessment
- Efficiency and value of health care services
- Oncology and hematology
- Cardiovascular disease and heart failure
- Neurology
- Influenza and other infectious diseases
- Geriatrics, specifically pharmacotherapy optimization in the elderly
Cooperation partners:
- Units at University of Basel, University Hospital Basel and
- Swiss Tropical and Public Health Institute
- Epidemiology, Biostatistics and Prevention Institute,
- University of Zürich
- Swiss Group for Clinical Cancer Research (SAKK)
- Swiss Medical Board
- Swiss Federal Office of Public Health
- Health Promotion Switzerland
- Health insurance providers & Industry
Recent/current key research projects:
- ENDOSCAPE: ENDOSCAPE: A clinically applicable non-viral
- gene delivery technology – EU HORIZON 2020
- OPERAM: Optimising PharmacothERApy in the Multimorbid elderly – EU HORIZON 2020
- SPEARHEAD: SwissPandemic AmR-Health Economy Awareness Detect – Innosuisse Flagship
- INSPIRE: Implementation of an integrated community-based care program for home-dwelling senior citizens – Swiss National Science Foundation, National Research Programme 74
- OPTICA: Optimising pharmacotherapy In the multimorbid elderly in primary care: a cluster randomised controlled trial – Swiss National Science Foundation, National Research Programme 74

